N(alpha)-Lauroyl-l-arginine ethylester monohydrochloride reduces bacterial growth in pasteurized milk
Abstract
Effective strategies for extending fluid milk product shelf-life by controlling bacterial growth are of economic interest to the dairy industry. To that end, the effects of addition of l-arginine, Nα-lauroyl ethylester monohydrochloride (LAE) on bacterial numbers in fluid milk products were measured. Specifically, LAE was added (125, 170, or 200 mg/L) to conventionally homogenized and pasteurized 3.25% fat chocolate or unflavored milk products. The treated milks and corresponding untreated controls were held at 6°C and plated on standard plate count agar within 24 h of processing and again at 7, 14, 17, and 21 d of storage. Bacterial counts in all unflavored milk samples treated with LAE remained below the Pasteurized Milk Ordinance limit for grade A pasteurized fluid milk of 4.3 log cfu/mL for the entire 21 d. Bacterial counts in unflavored samples containing 170 and 200 mg/L of LAE were significantly lower than those in the untreated unflavored milk at d 17 and 21 postprocessing. Specifically, bacterial counts in the milk treated with 200 mg/L of LAE were 5.77 log cfu/mL lower than in untreated milk at 21 d postprocessing. Bacterial counts in chocolate milk treated with 200 mg/L of LAE were significantly lower than those in the untreated chocolate milk at d 14, 17, and 21. In chocolate milk treated with 200 mg/L of LAE, bacterial counts were 0.9 log cfu/mL lower than in the untreated milk at 21 d postprocessing. Our results show that addition of LAE to milk can reduce bacterial growth. Addition of LAE is more effective at controlling bacterial growth in unflavored milk than in chocolate milk.
In recent years, the dairy industry has made strides in improving the quality of commercial fluid milk products (Carey et al., 2005). However, the highly competitive nature of the overall US beverage industry (IDFA, 2007) underscores the need for the dairy industry to employ innovative methods to ensure retention and possible growth of the fluid milk market share. Specifically, to compete with the rapidly expanding market share enjoyed by such products as shelf-stable bottled water and fruit juice-based beverages (US Census Bureau, 2008), fluid milk processors are striving to further improve product quality and extend shelf life. The most important factor limiting the shelf life of conventionally pasteurized fluid milk is bacterial growth. We hypothesized that use of a "generally recognized as safe" (GRAS) antimicrobial in fluid milk might provide an effective means of extending pasteurized product shelf life by controlling bacterial growth.
l-Arginine, Nα-lauroyl ethylester monohydrochloride (LAE) is a novel antimicrobial substance derived from lauric acid and arginine. It has been demonstrated as an effective antimicrobial against a variety of microbes. Rodriguez et al., 2004 examined the effect of LAE on Salmonella typhimurium and Staphylococcus aureus. In combination, transmission electron microscopy, fluorescence microscopy, flow cytometry, and ion-flux tests showed that LAE disrupts the structure of the cell membrane and consequently, membrane potential, which results in bacterial cell death. Specifically, the compound disrupts the lipid bilayer in the bacterial membrane, thereby interrupting metabolic processes and inhibiting cellular proliferation (Bakal and Diaz, 2005). Animal toxicological studies (Ruckman et al., 2004) showed that LAE has low acute toxicity, with systemic No Observable Adverse Effect Levels (NOAEL) established at 15,000 mg/L. In addition, mammalian metabolic studies have shown that LAE is metabolized into the amino acid arginine, which is ultimately broken down into CO2 and urea (Ruckman et al., 2004). In 2005, the FDA granted GRAS status to LAE for use as an antimicrobial in more than 20 foods, including meat and poultry products. Research by Luchansky et al., 2005 showed that Listeria monocytogenes populations inoculated onto the surface of ready-to-eat ham were reduced 2.9, 4.6, or 5.12 log cfu/mL when the surfaces had been treated with 4, 6, or 8 mL of a 5% LAE solution, respectively. l-Arginine, Nα-lauroyl ethylester monohydrochloride is not currently approved for use in dairy products (USDA, 2008c).
The objective of this study was to determine if addition of LAE to flavored and unflavored fluid milk products at levels currently approved for use in various food products would result in extension of product shelf life relative to that of comparable untreated products. The US Pasteurized Milk Ordinance (FDA, 2007) specifies a bacterial limit of 20,000 cfu/mL for grade A pasteurized milk while the milk is offered for sale; therefore, for the purpose of our study, "shelf life" was defined as the number of days postpasteurization that a fluid milk product can be held under refrigerated storage (6°C) before reaching 20,000 cfu of bacteria/mL.
Samples of commercially processed homogenized and pasteurized chocolate and unflavored fluid milk products that had been packaged in paperboard gable-top containers (polyethylene/paperboard/polyethylene) were obtained on processing day on each of 3 independent occasions from the Cornell University Dairy processing facility (Ithaca, NY). For each of the 3 trials, 946 mL (1 quart) of 3.25% fat unflavored homogenized milk and of 3.25% fat chocolate milk were obtained. Process temperatures were 79.5°C for 23 s for the unflavored milk and 82°C for 23 s for chocolate milk. Samples were collected and handled according to Standard Methods for the Examination of Dairy Products (SMEDP; Laird et al., 2004). A commercially prepared 10% LAE solution (Mirenat-N) was obtained from A&B Ingredients (Fairfield, NJ).
To create subsamples from the same product for testing at 7, 14, 17, and 21 d postprocessing, each milk sample was shaken as described in SMEDP and then the product was distributed aseptically among 4 sterile Pyrex bottles (Corning Inc., Corning, NY) with screw caps. The following treatments were prepared for both the chocolate and unflavored milk samples: a control (no LAE), 100 mL of milk with 125 mg/L of LAE, 100 mL of milk with 170 mg/L of LAE, and 100 mL of milk with 200 mg/L of LAE. As 200 mg/L of LAE was the upper limit suggested by the manufacturer (A&B Ingredients), it was chosen as the maximum LAE concentration for this study. The samples were held at 6°C and pour plated for bacterial enumeration according to SMEDP (Laird et al., 2004) within 24 h of processing, and at 7, 14, 17, and 21 d postprocessing. Bacterial colonies on the plates were counted after 48 h of incubation at 32°C. All standard plate count data were log10-transformed. Dunnett's method of statistical analysis was performed using JMP software (SAS Institute, Cary, NC) to compare bacterial counts in the treated samples to those in the control sample in each group.
The effects of different concentrations of LAE on bacterial numbers in unflavored and chocolate milk products were evaluated over a 21-d period (Figures 1 and 2). Overall, our data show that LAE limited bacterial growth and extended the shelf life of unflavored milk. Bacterial counts in the unflavored milk without LAE were <2 log cfu/mL immediately postprocessing and increased to >7 log cfu/mL after 21 d of storage. In the unflavored milk treated with 125, 170, or 200 mg/L of LAE, bacterial counts reached 3.64, 2.65, and 1.43 log cfu/mL respectively after 21 d of storage. Bacterial counts in unflavored milk treated with 200 mg/L of LAE were 5.77 log cfu/mL lower than in untreated milk at 21 d postprocessing. Bacterial counts in unflavored samples containing 170 and 200 mg/L of LAE were significantly lower (P < 0.05) than those in the untreated milk at d 17 and 21.
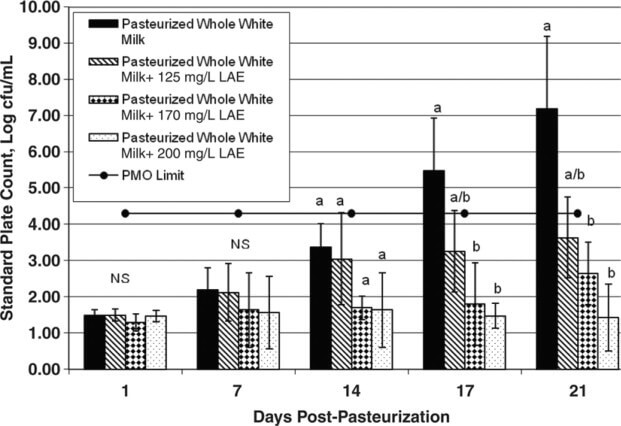
Figure 1 Mean standard plate count for pasteurized unflavored milk treated with l-arginine, Nα-lauroyl ethylester monohydrochloride (LAE; 0, 125, 170, or 200 mg/L) and held at 6°C for up to 21 d. The horizontal line at 4.3 log cfu/mL represents the Pasteurized Milk Ordinance (PMO) limit of 20,000 cfu/mL for pasteurized fluid milk. Error bars represent ±1 SD from the mean of data collected from 3 independent experiments. a,bDifferent letters within the same graph indicate statistically significant differences in bacterial numbers (P < 0.05) by Dunnett's method of statistical analysis on log10-transformed data.
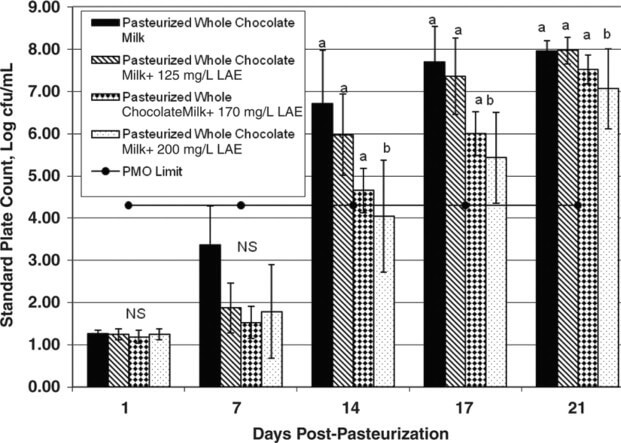
Figure 2 Mean standard plate count for pasteurized chocolate milk treated with l-arginine, Nα-lauroyl ethylester monohydrochloride (LAE; 0, 125, 170, or 200 mg/L) and held at 6°C for up to 21 d. The horizontal line at 4.3 log cfu/mL represents the Pasteurized Milk Ordinance (PMO) limit of 20,000 cfu/mL for pasteurized fluid milk. Error bars represent ±1 SD from the mean of data collected from 3 independent experiments. a,bDifferent letters within the same graph indicate statistically significant differences in bacterial numbers (P < 0.05 or P < 0.075 for 200 mg/L of LAE on d 14) by Dunnett's method of statistical analysis on log10-transformed data.
Bacterial counts in chocolate milk without LAE were <2 log cfu/mL immediately after processing, but increased to nearly 8 log cfu/mL after 21 d of storage. Chocolate milk treated with 125, 170, or 200 mg/L of LAE had bacterial counts of 7.96, 7.44, and 7.06 log cfu/mL respectively, after 21 d of storage. Bacterial counts in chocolate milk treated with 200 mg/L of LAE were 0.9 log cfu/mL lower than those in the untreated milk at 21 d postprocessing. Bacterial counts in the chocolate milk samples treated with 200 mg/L of LAE were significantly lower than in the control samples (P < 0.05) at d 17 and 21, with a weakly significant difference (P < 0.075) between the control and the chocolate sample containing 200 mg/L of LAE on d 14. There were no significant differences between the samples with 170 and 125 mg/L of LAE and the controls on any test day (P > 0.05).
Higher bacterial numbers in matched samples of chocolate and unflavored milk have been documented previously by Douglas et al., 2000. Specifically, pasteurized chocolate milk had a greater relative increase in bacterial numbers over 21 d of shelf life when compared with paired pasteurized unflavored milk. The chocolate powder that had been added to the milk was implicated as promoting more rapid microbial growth in the chocolate milk relative to that in the unflavored milk. Our results are consistent with the findings from this previous study. In the present study, counts in the chocolate control samples (without LAE) reached 20,000 cfu/mL between 10 and 14 d postprocessing, whereas counts in the unflavored control milk reached 20,000 cfu/mL between 14 and 17 d postprocessing. The addition of LAE more effectively retarded bacterial growth in unflavored milk than in chocolate milk. It is possible that the reduced effectiveness of LAE in chocolate milk is because of the presence of stabilizers in the chocolate powder (e.g., carrageenan), as the presence of stabilizers has been implicated in decreasing the effectiveness of LAE in retarding bacterial growth (Gil Bakal, A&B Ingredients, Fairfield, NJ; personal communication).
The cost of adding 200 mg/L of LAE to fluid milk would be approximately $0.30 per gallon of finished product (as of August 2008). Therefore, with the average retail price of a gallon of whole milk at $3.816 in 2008, the addition of LAE would result in at least a 9.6% increase in retail price. The addition of LAE to milk will also affect the labeling of milk products. According to the USDA, 2008a, USDA, 2008b), the addition of LAE to fluid milk would require labeling the product as "milk" with the addition of the common name of the ingredient and a statement describing the function of the ingredient, such as "preservative," or "to retard spoilage" rather than simply labeling the product as "milk." Consumer perspectives on this proposed designation would need to be assessed to gauge acceptance of this additive.
The present study did not assess the sensory consequences of LAE addition on product flavor or acceptability. Future testing of the effects of LAE on sensory characteristics of milk will be needed. Although the addition of the antimicrobial LAE to chocolate and unflavored milk was effective in retarding bacterial growth, it was considerably more effective in unflavored milk than in chocolate milk. Our results suggest a possible role for GRAS antimicrobials such as LAE for use in the dairy industry as a way to extend product shelf life.
How Can We Help?
We are here to help you with development of new and improved food products. Our technical service and sales teams can assist you in choosing the right ingredients best suited for your applications.
Product CatalogContact Us
