The Lowdown on Lauric Arginate
Food antimicrobial hammers away at plasma membrane, disrupting a pathogen's metabolic process
To request samples, visit CytoGUARD LA.
As more and more food products are produced on a large industrial scale and distributed over extensive geographic areas, the concerns regarding quality and food safety throughout the shelf life of the product have become more and more critical. Effective control of bacterial growth is a constant concern due to the risk of spoilage and food-borne disease.
The federal government continually estimates that 76 million cases of foodborne illnesses occur each year in the U.S. alone, resulting in 300,000 hospitalizations and 5,000 deaths annually. The populations most at risk are the very young, those who have a chronic illness, and those with damaged immune systems. Bacterial pathogens are the most commonly identified cause of food-borne illness. Some of these pathogens include Campylobacter, Salmonella, Clostridium, Escherichia and Staphylococus. Deaths have occurred due to bacterial pathogens, with Salmonella and Listeria topping the list.
Lauric arginate is a novel antimicrobial compound derivative of lauric acid, Larginine and ethanol, all naturally occurring substances. The molecule was first synthesized by the CSIC (Higher Council of Scientific Research) in Barcelona in 1984. It was then patented and commercialized by the Vedeqsa Lamirsa Group and distributed in the U.S. through A&B Ingredients. Its most notable features are:
- Broad spectrum of antimicrobial efficacy;
- High partition coefficient (>10), means the product concentrates in the water phase of products, where most bacterial action occurs;
- Activity over a wide pH range (3 to 7);
- Safe ingredient: LAE is hydrolyzed in the human body by chemical and metabolic pathways, which quickly break the molecule into its natural components;
- GRAS Status: Granted by a panel of FDA experts in the U.S. through scientific procedures set forth under 21 CFR §170.30(b).
Antimicrobial Activity
Its low toxicity in conjunction with its remarkable antimicrobial properties, make LAE a valuable tool for the food industry for improved safety and product quality.
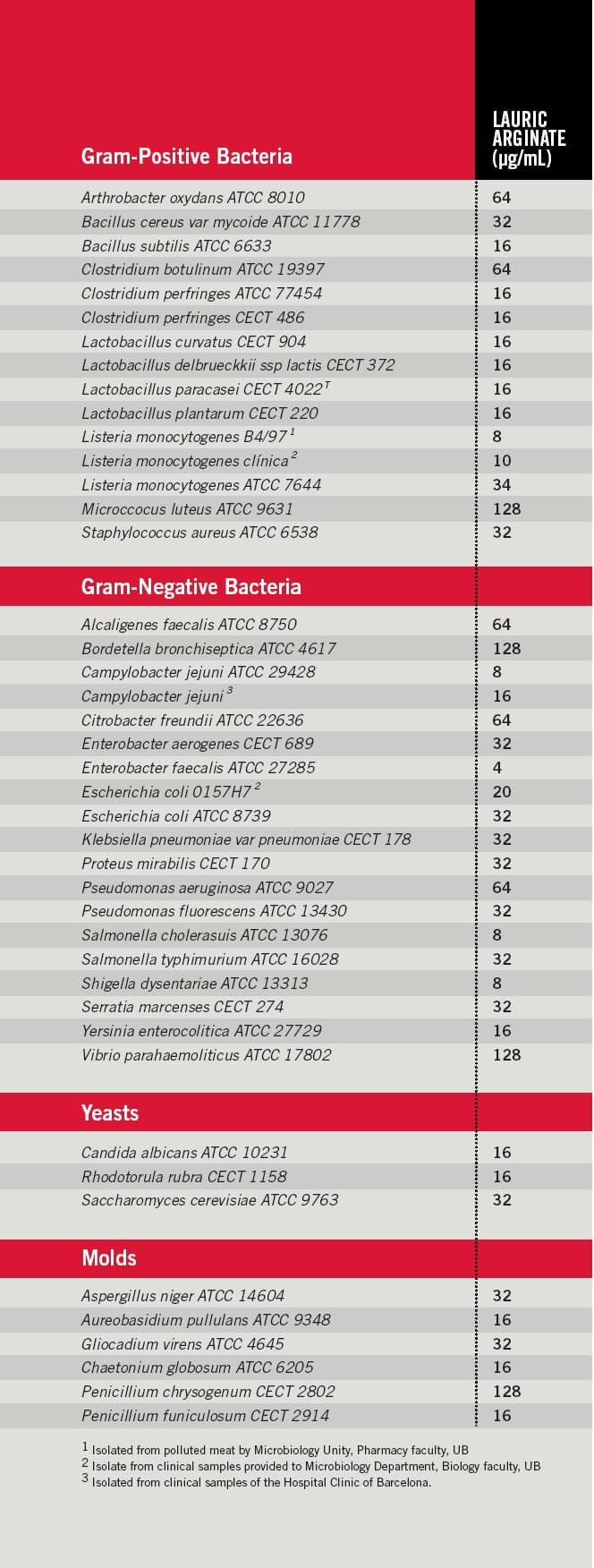
The following tables (p. 58) show the minimal inhibitory concentrations of LAE versus different types of microorganisms, mostly pathogens, which give insight into compound potency and activity range. These results were obtained by an independent laboratory from the faculty of pharmacy and biology at the University of Barcelona.
Action Mechanism
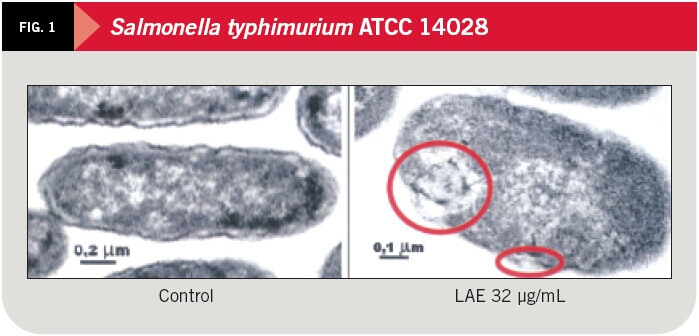
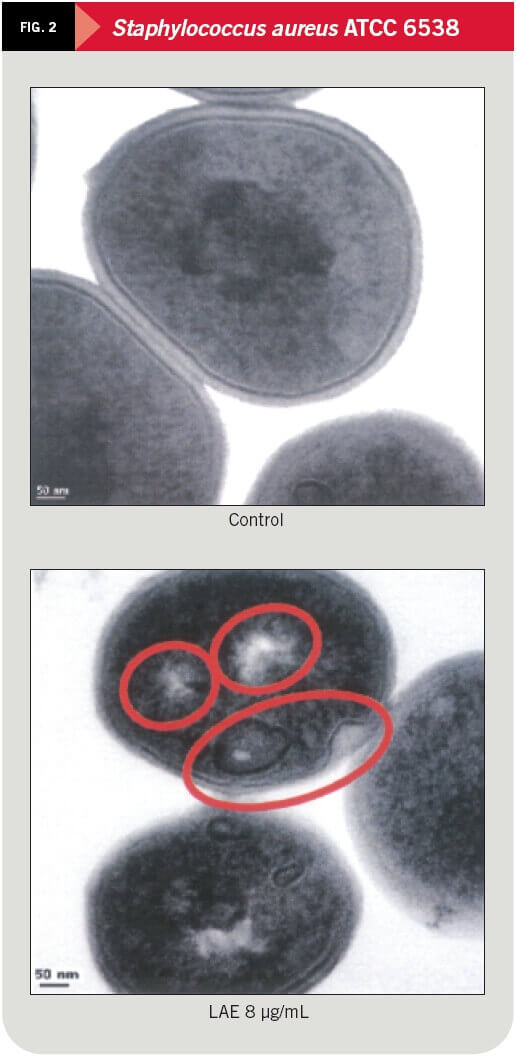
The antimicrobial properties of LAE are derived from its action on the cytoplasmic membranes of microorganisms. LAE causes a disruption/instability of plasma membrane lipid bilayer, altering the metabolic process and detaining the cellular cycle. No cellular lysis has been observed in any case in bacteria cells exposed to usual doses of LAE.
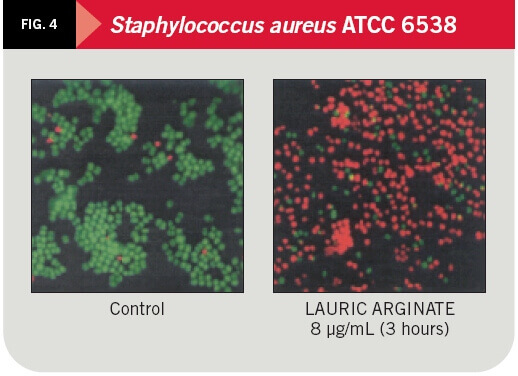
The effect of LAE on gram-negative microorganisms (Salmonella typhimurium ATCC 14028) and gram-positive (Staphylococcus aureus ATCC 6538) was studied at inhibition concentrations of 32 y 8 ºg/mL respectively, by the pharmacy faculty at the University of Barcelona and recently published in the Journal of Applied Microbiology.
By means of electronic transmitting microscopy the alterations caused by LAE on the bacteria cytoplasmic membrane were studied and can be seen clearly in figures 1, 2 and 3.
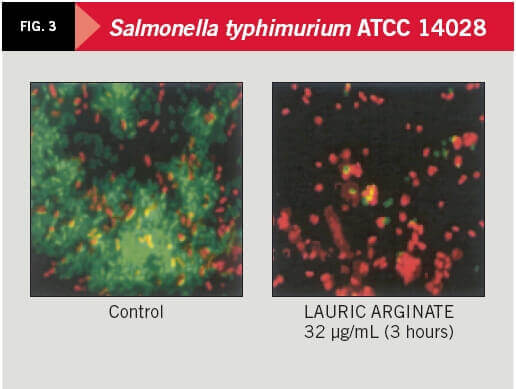
The action level on bacterial populations was analyzed by means of fluorescence microscopy and flux citometry, based on nucleic acid staining with two fluorescent pigments: first SYTO-13 (green), marking all cells, and then propidium iodide (red) which replaces the previous one only in damaged cells. LAE was able to act on 91 percent of the cell population of S. typhimurium and 45 percent of S. aureus population after 30 minutes of contact and 96 percent and 55 percent, respectively, after three hours of contact.
Finally, potassium and proton trans-membrane ionic flux was determined after 30 minute contact showing a potassium flux increment for S. aureus and S. typhimurium, as well as a decrease in the proton flux.
Both experiments indicate a clear alteration of the membrane potential as a direct consequence of the structural modification induced by LAE on the bacterial membrane.
Toxicological Safety, Human Studies
For many consumers, the claim "no preservative added" often gives the false impression of improved quality. However, the proper use of a safe food preservative can dramatically improve the safety and quality of foodstuff.
A set of toxicological experiments, carried out by Huntingdon Life Science, Ltd. , assessed the safety of LAE in different areas: Metabolism, mutagenicicity, subchronic, chronic, reproduction and developmental toxicity and acute toxicity. The results obtained with these studies have been published in the scientific journal Food Chemical and Toxicology. Finally, these studies were complemented with human studies.
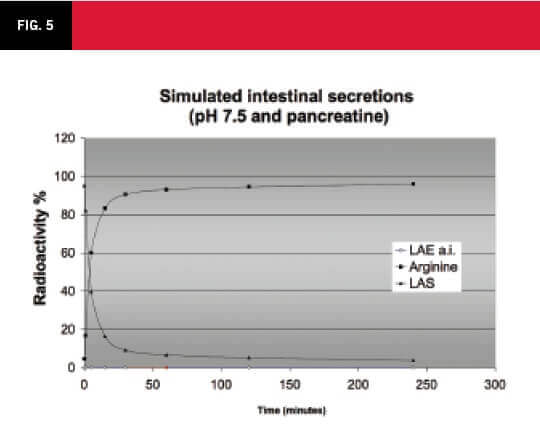
The conclusions of these studies were that LAE is safe and can be used for human consumption. The metabolism of LAE, after its ingestion, the potential points of its degradation (intestines, liver and plasma) and its pharmacokinetics, are shown in the Figure 5. The graph shows the degradation of LAE in simulated intestinal secretions with pancreatine presence at pH 7.5.
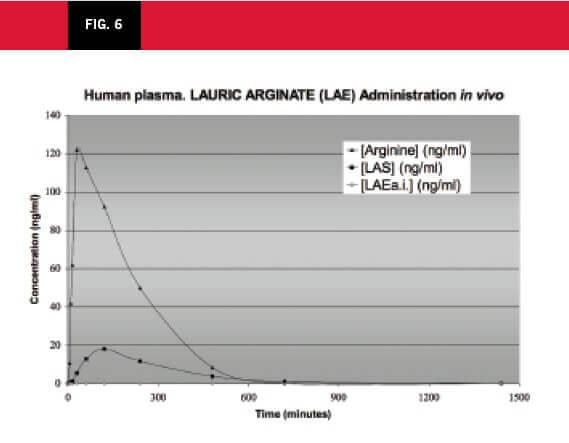
The LAE is rapidly degraded to LAS (an intermediary) and subsequently to arginine in the intestinal secretions with the presence of pancreatine. LAE and LAS degradations were also studied in plasma and human hepatocytes (See Figure 6).
Volunteers received an oral dose of LAE at 2.5 mg/kg bw. The following figure demonstrates the rapid degradation of LAE in blood plasma samples.
Conclusions
Lauric arginate is rapidly degraded to LAS which is subsequently degraded to Arginine and finally to endogenous compounds.
Lauric arginate does not produce mutagenic effects and has no adverse effects on fertility, reproduction rate and fetus development. The results of sub-chronic toxicity experiments established a NOAEL value at 15,000 ppm (1143 and 1286 mg/kg bw/day for males and females, respectively.)
Lauric arginate and its hydrolysis products have been sufficiently characterized to assure safe human consumption at the dose level established.
Food Applications: Treatment in Cured Ham
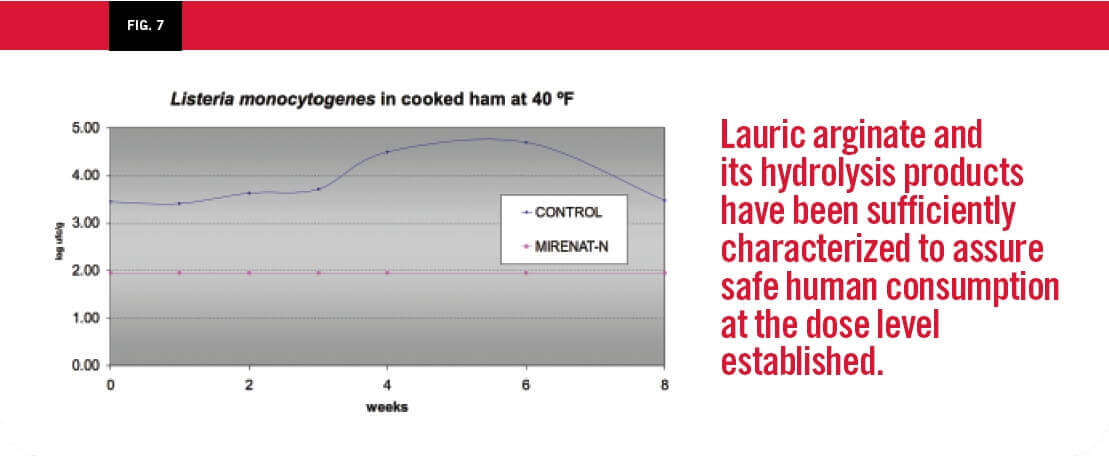
Figure 7 illustrates the use of lauric arginate applied to cured ham and cooked ham through a bath. The first table was a study of the shelf life of a cured ham, measuring aerobic plate count. The second study was examining the effect of a bath treatment on cooked ham inoculated with 4 log of Listeria monocytogenes.
LAE is effective for controlling pathogenic bacteria such as L Monocytogenes in cooked meats. It extends shelf life of meat products by controlling proliferation of decay flora and improves the product appearance during refrigerated storage due to its activity against lactic acid bacteria, which are the main agents in deterioration of organoleptic conditions of food products.
Copyrighted material, reprinted with permission from Food Quality Magazine.
How Can We Help?
We are here to help you with development of new and improved food products. Our technical service and sales teams can assist you in choosing the right ingredients best suited for your applications.
Product CatalogContact Us
